A reversible cycle is an ideal hypothetical cycle in which all the processes constituting the cycle are reversible. Carnot cycle is the reversible cycle. For a stationary system, as in a piston and cylinder machine, the cycle consists of the following four successive processes.
1. A reversible isothermal process in which heat Q1 enters the system at t1 reversibly from a constant temperature source at t1 when the cylinder cover is in contact with the diathermic cover A. The internal Energy of the system increases.
From the first Law,
Q1 = U2 - U1 + W(1-2)
(for an ideal gas only, U1 = U2)
2. A reversible Adiabiatic Process in which the diathermic cover A is replaced by the adiabiatic Cover B, and work We is done by the system adiabatically and reversibly at the expense of its inernal energy, and the temperature of the system decreases from t1 to t2 .
Using the first law ,
0 = U3 - U2 + W(2-3)
3. A reversible isothermal process in which B is replaced by A and heat Q2 leaves the system at t2 to a constant temperature sink at t2 reversibly, and the internal energy of the system further decreases.
from the first law,
-Q2 = U4 - U3 - W(3-4)
only for an ideal gas,
4. A reversible adiabatic process in which B again replaces A , and work Wp is done upon the system reversibly and adiabatically, and the internal energy of the system increases and the temperature rises from t2 to t1.
Applying the first law,
0 = U1 - U4 - W (4-1)
Two reversible isotherms and two reversible adiabatics constitute a Carnot cycle, which is represented in p-v coordinates
Q1 - Q2 = ( W(1-2) - W (2-3)) - ( W(3-4) + W(4-1) )
Q net = W net
A cyclic heat engine operating under carnot cycle is called a Carnot Heat engine.
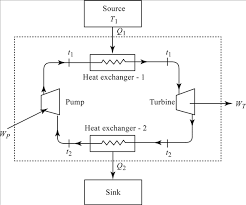
For a steady flow cycle ,


Comments
Post a Comment